Xôi lạc chấm tivi, trực tiếp bóng đá trực tuyến, xoi lac tivi tốc độ cao
Xoilac TV luôn được đánh giá là một kênh trực tiếp bóng đá chất lượng, theo dõi ngay bài viết này để hiểu thêm thông tin về nền tảng này nhé.
Ngày 20/04/2024
Xoilac tv được ra đời chủ yếu nhằm mục đích là phục vụ nhu cầu xem trực tiếp các giải bóng đá của những người hâm mộ thể thao trên thế giới. Tại đây, bạn có thể xem được tất cả các giải đấu từ nhỏ cho đến lớn. Không chỉ vậy, hệ thống của xoilac tv còn vô cùng chất lượng và có rất nhiều thông tin thú vị.
Xem trực tiếp các giải đấu bóng đá miễn phí cùng xoilac tv
Xoilac tv hiện là một trang web bóng đá trực tuyến được nhiều người đánh giá rất cao. Đây còn được xem là nơi mà bạn có thể thỏa sức theo dõi các giải đấu bóng đá với chất lượng cao. Cùng với đó, bạn còn có thể tham khảo các thông tin khác về bóng đá mỗi ngày tại xoilac tv.
Sứ mệnh của xoilac tv không chỉ đơn giản là mang tới các thông tin mà còn mang tới các trải nghiệm và cái nhìn của bóng đá đến với tất cả mọi người trên toàn thế giới. Sự ra đời của xoilac tv đã tạo nên một môi trường tương tác đầy sống động cùng với các thông tin đa dạng về bóng đá. Đó là nơi, các tin tức hay khoảnh khắc đáng nhớ của các giải đấu được ghi lại. Cũng là nơi, người hâm mộ bóng đá có được cái nhìn màu sắc về các trận đấu.

Hơn nữa, tại xoilac tv người yêu bóng đá còn có thể tham gia vào cộng đồng người hâm mộ bóng đá đầy nhiệt huyết. Mọi thành viên trong môi trường đó, họ có thể chia sẻ cảm xúc, ý kiến của mình về các trận đấu, cầu thủ hoặc cả về lối chơi, chiến thuật. Sự kết nối cộng đồng của xoilac tv bằng cách tạo ra một không gian cho những điều về bóng đá cũng đã thúc đẩy sự gắn kết của một cộng đồng trên toàn thế giới.
Có lẽ, xoilac tv sẽ trở thành một môi trường không chỉ thúc đẩy sự hiểu biết, khám phá của mọi người mà còn là nơi mang những tâm hồn cùng chung sở thích đến lại gần nhau hơn.
Xoilac tv cung cấp các tính năng trải nghiệm gì?
Xoilac tv được biết tới là nơi cung cấp các dịch vụ về các giải đấu bóng đá lớn nhỏ. Nhưng cạnh đó, địa chỉ này còn là nơi cung cấp rất nhiều các tính năng trải nghiệm phục vụ nhu cầu của mọi người. Tất cả các tính năng của xoilac tv sẽ được cập nhật ở phần dưới đây:
Tin tức trực tiếp bóng đá xoilac tv
Xoilac tv là địa chỉ cung cấp các dịch vụ cũng như các tin tức trực tiếp về các giải đấu bóng đá hàng đầu thế giới. Mỗi ngày, các chuyên viên và đội ngũ làm việc sẽ cập nhật lên website các thông tin mới nhất về bóng đá. Do đó, các bạn có thể theo dõi địa chỉ và cập nhập tin tức bóng đá một cách rõ ràng và chi tiết nhất.
Bên cạnh những tin tức về các giải đấu, xoilac tv còn cung cấp các tin tức như cầu thủ, chuyển nhượng và huấn luyện viên,.... Các chủ đề đa dạng hứa hẹn sẽ có tại xoilac tv để dành cho bạn.
Lịch thi đấu bóng đá sớm nhất
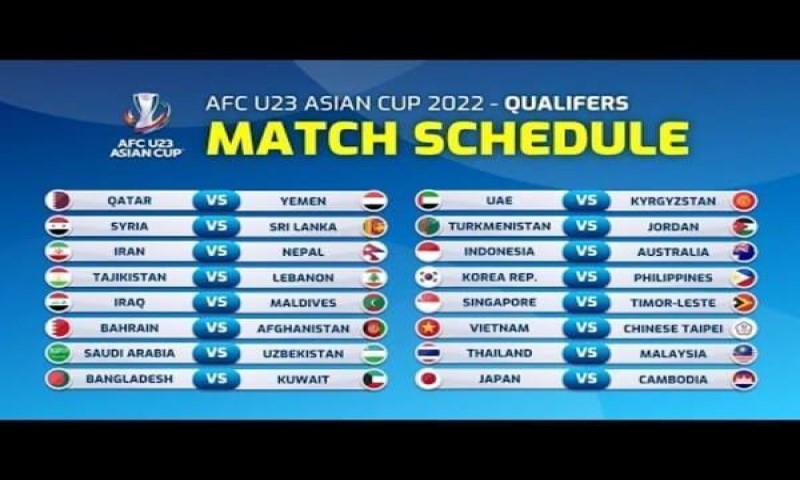
Ngoài việc phát sóng trực tiếp các trận đấu trên nền tảng của mình, xoilac tv còn cung cấp cả lịch thi đấu của từng giải đấu từ trong nước đến quốc tế. Thông qua đó, người hâm mộ bóng đá có thể dễ dàng cập nhật thông tin về thời gian, địa điểm diễn ra các trận đấu và các vấn đề liên quan đến giải đấu mà họ muốn xem. Có thể nói, đây cũng là tính năng mà xoilac tv muốn giúp cho người hâm mộ có được các kế hoạch xem bóng đá tốt nhất.
Ngoài ra, các thông tin liên quan đến đội hình ra sân, phong đội hay thành tích hiện tại,... của các đội bóng cũng sẽ được cập nhật chi tiết tại trang chủ của xoilac tv.
Phân tích trận đấu và bình luận
Xoilac tv còn là một nền tảng giúp cho người hâm mộ bóng đá có được những kiến thức về các trận đấu. Trang web sẽ phân tích chi tiết, phân tích chất lượng của từng trận đấu, chiến thuật chơi của từng đội để giúp cho người xem nắm bắt được tình tình hiện tại.
Các bình luận viên và các chuyên gia bóng đá của xoilac tv là những người hiểu biết sâu rộng về môn thể thao vua này. Do đó, các bạn có thể yên tâm khi họ mang đến các thông tin, phân tích các chiến thuật hay bình luận các sự kiện luôn ở các góc độ khách quan.
Với xoilac tv, người hâm mộ bóng đá không chỉ là người xem mà còn là những người hiểu biết rõ về bóng đá. Cái nhìn từ xoilac tv chỉ là những góc nhìn nhỏ và sự tham gia phân tích của người chơi mới mang lại cái nhìn rộng hơn, sâu sắc hơn.
Kết quả của các giải đấu bóng đá lớn nhỏ
Tại xoilac tv, tất cả các trận đấu diễn ra trong vòng 24h đều sẽ được trang chủ cập nhập kết quả chi tiết và đầy đủ. Ngoài ra, các bạn còn có thể nắm được các thông tin như cầu thủ ghi bàn, tỷ số, số thẻ phạt, thời gian ghi bàn, ném biên,... của các giải đấu lớn nhỏ khác nhau.
Tỷ lệ kèo xoilac tv
Trang web xoilac tv còn là nơi cung cấp và cập nhật bảng tỷ lệ kèo cược của mọi trận đấu lớn nhỏ diễn ra hàng ngày trong nước và trên thế giới. Thông thường, các chuyên gia phân tích sẽ nhận định các bảng tỷ lệ kèo rất chính xác. Vì thế, anh em có thể dễ dàng sử dụng bảng kèo để soi kèo các trận đấu mà mình mong muốn với tỷ lệ chuẩn xác nhất.
Trực tiếp soi kèo cùng xoilac tv

Đối với những người thích theo dõi trực tiếp soi kèo và đặt cược bóng đá, thì đến xoilac tv là điều tuyệt vời đúng đắn. Tại đây, bạn sẽ được chia sẻ những kinh nghiệm soi kèo đỉnh cao từ các chuyên gia và bóng thủ. Sau một thời gian tham khảo, bạn hãy yên tâm trình độ đặt cược của bạn cho các trận đấu bóng đá sẽ tăng vọt.
Lý do nên chọn xoilac tv để xem trực tiếp bóng đá
Xoilac tv là địa chỉ xem trực tiếp bóng đá được rất nhiều người ưa chuộng. Tại sao lại như thế? Câu trả lời sẽ có ở ngay phần dưới đây:
Giao diện của xoilac tv chất lượng và đẹp mắt
Khi tham gia vào xoilac tv, bạn sẽ thật sự ấn tượng với giao diện. Bởi thiết kế giao diện của xoilac tv rất đẹp mặt và thông minh. Bởi, trang web này được xây dựng và phát triển từ nhờ rất nhiều những khảo sát và ý kiến của hàng nghìn người hâm mộ xem trực tuyến các giải đấu bóng đá. Do đó, đội ngũ thiết kế của địa chỉ đã tạo nên một giao diện với thiết kế đầy đủ mọi tính năng đáp ứng nhu cầu của từng người dùng trong thời điểm hiện tại.
Sự kết hợp hài hòa màu sắc trong giao diện của xoilac tv cũng giúp cho địa chỉ này thêm phần ấn tượng hơn với các thành viên mới. Ngoài ra, các danh mục và thông tin quan trọng cũng đã được lưu chú và sắp xếp vô cùng khoa học. Điều này, có lẽ đã giúp cho tất cả mọi người có thể đến với xoilac tv và tìm kiếm thông tin dễ dàng.
Xoilac tv cung các các video full HD sắc nét

Cũng như nhiều trang web cung cấp các thông tin về bóng đá, xoilac tv luôn đề cao chất lượng hình ảnh và video. Chính vì thế, họ rất chú trọng việc xây dựng và nâng cao chất lượng các video được phát ra. Có thể nói, đây chính là một điểm cộng của xoilac tv ngày càng hiểu tâm lý của người hâm mộ. Họ trở thành cái tên được nhiều người săn tìm cũng là vì thế.
Nhưng xoilac tv còn ấn tượng hơn khi:
- Áp dụng công nghệ xử lý thông tin hiện đại vào các trận phát sóng trực tiếp bóng đá, giúp cho tốc độ truyền tải các video mượt hơn và nhanh chóng hơn. Người xem cũng được theo dõi các trận đấu bóng đá trực tiếp được tuyệt vời và trọn vẹn nhất.
- Chất lượng âm thanh, hình ảnh tại xoilac tv cũng rất được chú trọng. Hình ảnh của tất cả các trận đấu đều có độ phân giải rất cao. Thêm vào đó, hệ thống âm thanh của web xoilac tv sống động, nâng cao được sự trải nghiệm tuyệt vời của các khách hàng. Từ đó, người hâm mộ bóng đá đã có được những giây phút tận hưởng bóng đá cực kỳ sắc nét và chân thực.
Xoilac tv phát sóng đa dạng các trận đấu và giải đấu lớn nhỏ

Xem trực tiếp bóng đá tại xoilac tv, bạn hoàn toàn được tận hưởng và chìm đắm trong những trận cầu gay cấn. Các giải đấu lớn nhỏ từ trong nước đến quốc tế sẽ được phát sóng tại xoilac tv một cách đầy đủ và chi tiết:
- Giải Ngoại hạng Anh (Premier League): Đây là một giải thi đấu bóng đá dành cho tất cả các CLB Anh, giải thi đấubóng đá cấp cao nhất trong hệ thống những giải đấu bóng đá của Anh và là một giải đấu chính trong hệ thống giải thi đấu bóng đá quốc gia.
- Giải La Liga: Giải thi đấu bóng đá vô địch của quốc gia Tây Ban Nha thường được gọi là giải La Liga, đây là một hạng đấu bóng đá rất chuyên nghiệp cao dành cho nam nằm trong hệ thống những giải bóng đá của Tây Ban Nha.
- Serie A: Đây là một giải đấu bóng đá chuyên nghiệp cao nhất trong hệ thống những giải đấu bóng đá của Ý do nhà TIM tài trợ. Tính đến nay thì giải đấu này đã có hơn 80 năm hoạt động nhận được ratas nhiều sự quan tâm của nhiều người.
- Bundesliga: Đây là giải thi đấu bóng đá vô địch quốc gia của Đức, thuộc hạng cấp cao nhất bóng đá của Đức.

- Euro: Đây là một giải đấu vô địch các đội tuyển của châu Âu nằm trong hệ thống của FIFA, bạn có thể đón xem các trận trực tiếp Euro ngay tại kênh truyền Mitom1 tv.
- UEFA Champion League: Đây là giải thi đấu bóng đá cấp CLB hàng năm đã được tổ chức bởi Liên đoàn bóng đá châu Âu dành cho những câu lạc bộ có thứ hạng cao nhất tại các giải đấu vô địch quốc gia của châu Âu.
- Europa League: Giải thi đấu bóng đá hàng năm này do Liên đoàn bóng đá châu Âu tổ chức thi đấu cho những câu lạc bộ giành được nhiều thứ hạng cao nhưng lại không giành được quyền để tham dự UEFA Champion League.
- World Cup: Giải thi đấu vô địch bóng đá thế giới được tổ chức 4 năm 1 lần cho tất cả các đội tuyển bóng đá của quốc gia với những nước thành viên của FIFA.
- V-League: Giải thi đấu bóng đá vô địch của Việt Nam là giải thi đấu bóng đá trực tiếp rất chuyên nghiệp của quốc gia Việt Nam, được Công ty Cổ phần Bóng đá chuyên nghiệp Việt Nam đến sáng lập và điều hành đến nay.
- Và 1 số giải thi đấu bóng đá nhỏ khác.
Trực tiếp bóng đá tại xoilac tv không chứa quảng cáo
Xoilac tv là địa chỉ mang tới thông tin và trải nghiệm hữu ích cho người hâm mộ bóng đá. Chính vì vậy, trong các trận đấu khi được phát trực tiếp tại đây sẽ không bao giờ được chèn quảng cáo hay các banner. Xoilac tv cam kết không bao giờ làm gián đoạn thời gian tận hưởng của người hâm mộ khi xem bóng đá tại đây. Bởi vậy, chuyên trang bóng đá luôn được nhiều người đánh giá rất cao cũng như có sự tăng vọt về mức độ tham gia tại đây ngày càng cao.
Hướng dẫn xem trực tiếp bóng đá cùng xoilac tv

Nếu bạn vẫn chưa biết cách sử dụng web xoilac tv để xem trực tiếp bóng đá thì hãy theo dõi phần thông tin dưới đây:
- Bước 1: Truy cập vào website xoilac tv
- Để truy cập vào web xoilac tv, người dùng có thể sử dụng trình duyệt web trên máy tính hoặc thiết bị di động. Hoặc các bạn cũng có thể tải ứng dụng riêng của xoilac tv trên các hệ điều hành IOS và Android. Tải ứng dụng này chỉ cần một vài bước đơn giản trên thiết bị di động. Tóm lại để tiếp cận vào xoilac tv, mọi người có thể sử dụng nhiều phương thức khác nhau linh hoạt và dễ dàng.
- Bước 2: Đăng ký tài khoản
- Để được phép xem các trận đấu thì người dùng phải đăng ký tài khoản. Để tạo tài khoản, người dùng chỉ cần cung cấp cho hệ thống xoilac tv các thông tin cơ bản như tên, email và mật khẩu. Khi có tài khoản, bạn sẽ được trải nghiệm rất nhiều các tiện ích và có những trải nghiệm tốt nhất tại đây.
- Bước 3: Chọn các giải đấu để xem các trận đấu
- Khi các bạn đã được duyệt tài khoản đăng ký thành công thì bạn sẽ biết các thông tin về các trận đấu dễ dàng hơn. Nếu bạn có một nhu cầu xem một trận đấu cụ thể nào đó, bạn có thể tìm kiếm nhanh chóng hơn rất nhiều.
- Bước 4: Xem trực tiếp trận đấu
Khi đã tìm thấy trận đấu ưng ý của mình trên nền tảng xoilac tv. Lúc này, bạn chỉ cần thao tác một vài nút là cánh cửa của những niềm vui sẽ đến. Trên màn hình sẽ xuất hiện những khoảnh khắc của các trận đấu giành riêng cho bạn.
Kết luận
Trên đây, chúng tôi đã cung cấp cho các bạn các thông tin về web bóng đá xoilac tv. Xoilac tv không chỉ là một nền tảng phát sóng trực tiếp bóng đá đơn thuần mà còn là một nơi mang đến cho bạn các kiến thức và niềm vui về bóng đá. Hãy lựa chọn xoilac tv là nền tảng yêu thích nhất nhé!